Leprechaun takes the guesswork out of your lentivirus production
Blog
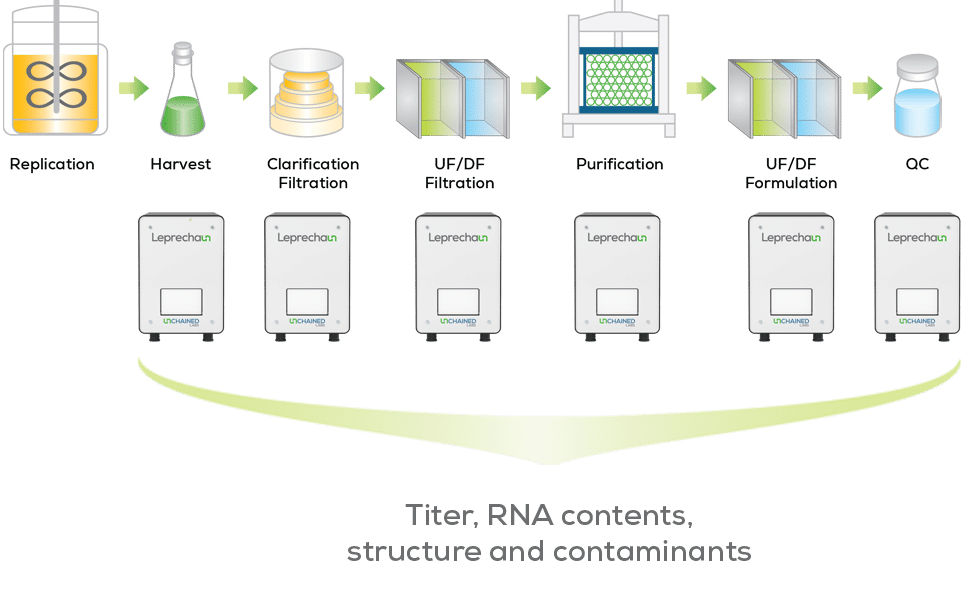
Lentivirus production and purification can feel like navigating a maze. It is a complex, multi-step process following a standardized framework of harvest, clarification, filtration, chromatographic separation, and final polishing. Each step is essential for ensuring a high yield and purity of intact lentivirus, but lentivirus production can be both time-consuming and costly. That’s why optimizing each step is crucial.
Struggling to see the whole lenti picture?
There are several established methods for performing lentivirus QC: ELISA ensures the presence of the viral capsid, qRT-PCR confirms the presence of RNA, and cell-based transduction assays confirm the functionality of the final product. However, each method has its limitations. They often lack information on virus structural information (all), fail to differentiate capsid and soluble p24 (ELISA), or take too long to deliver results (transduction assay). Effective process optimization hinges on the ability to accurately quantify the content of lentivirus preps before and after each process step. The inabililty of established analytical methods to provide information on crude samples limits their suitability for process development optimization. Moreover, these analytical methods focus exclusively on lentiviral components within a sample. As the cell therapy market evolves, there is a growing need to monitor the titer and functional impact of non-viral contaminants within the lentivirus prep, which may interfere with viral function.
Streamline lentivirus production and purification with Leprechaun
With Leprechaun, you no longer need to worry about sample purity. Developed for total lentivirus analysis, Leprechaun helps streamline QC and the development of the lentivirus production process. The platform provides complete biophysical characterization of any lentivirus product by measuring intact lentivirus titer through detection of viral capsid and RNA, while also quantifying the concentration of contaminating particles, such as extracellular vesicles (EVs).

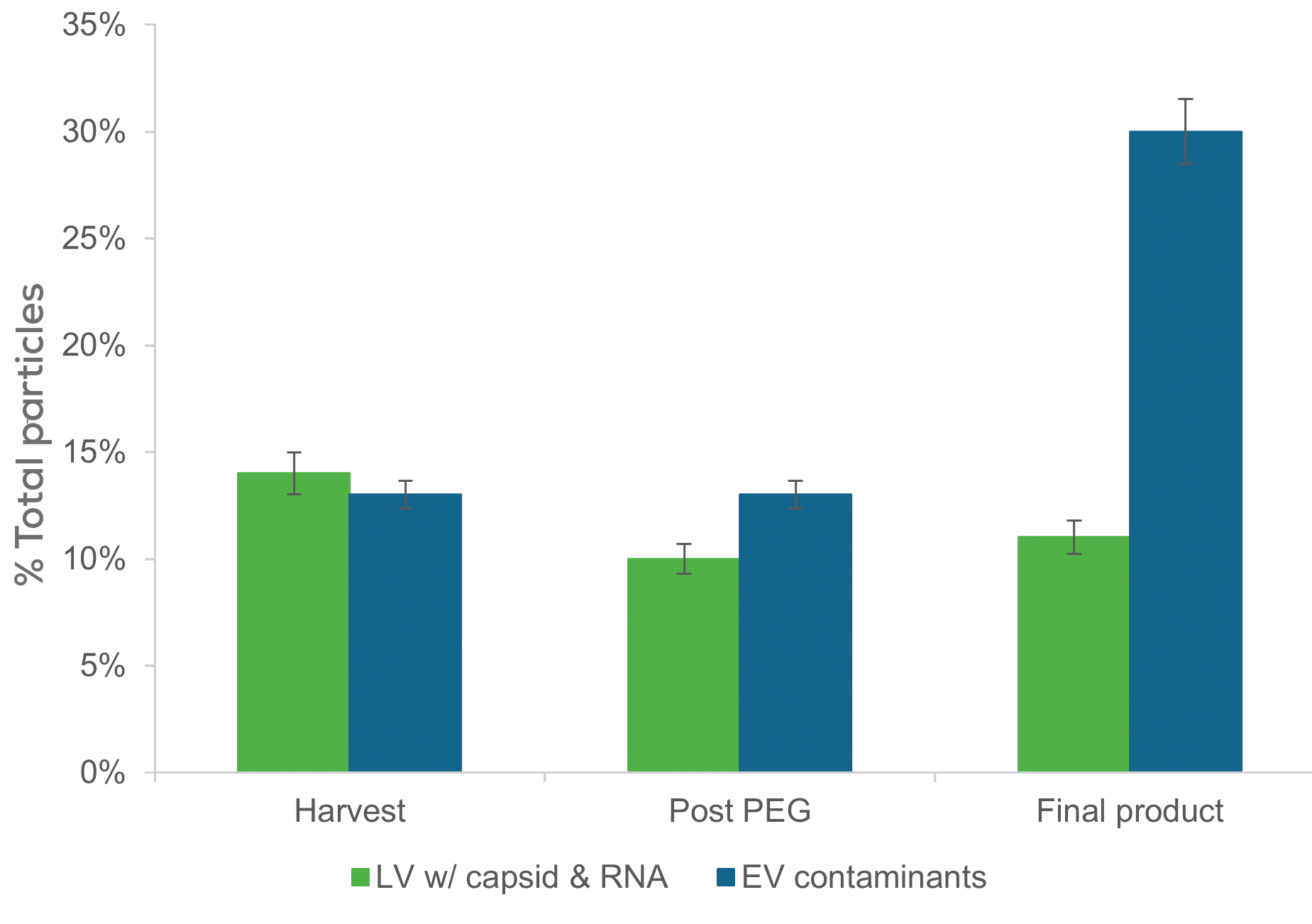
Track down the good stuff at every stage
By monitoring intact lentivirus titer and contaminant concentration in parallel, before and after each process step, you can optimize every step in the purification process to ensure the most structurally intact lentivirus particles. Plus, with our Leprechaun kits, you can identify which batches have the highest quality material at harvest with the RNA kit, delve into purity analysis with our contaminant kit, or customize the assay or capture marker using our Flex Kit. Leprechaun is compatible with both VSV-G and non-VSVG pseudotyped lentivirus.
Conclusion
With Leprechaun, we take the guesswork out of your lentivirus production, giving you clear, comprehensive insights every step of the way. So, why not streamline your lentivirus production process and ensure the highest quality lentivirus particles with Leprechaun? Your research deserves nothing less.

Leprechaun
无论样品的纯度如何,Leprechaun都可以准确地检测出病毒和外泌体的滴度和结构。Leprechaun可以连续三次检测慢病毒颗粒,确保慢病毒拥有合适的大小、包膜蛋白和p24衣壳蛋白以及含有RNA。快来与Leprechaun一起揭开外泌体和病毒表型的奥秘吧!